Art. 67 (2) Patent Act: extension for inventions underlying approval procedure
Following the decision of the Supreme Court of Japan (Decision of the Third Petty Bench of the Supreme Court, Nov. 17, 2015 (2014 (Gyo Hi) No. 356)), the Japanese Examination Guidelines for Extension of Patent Terms were revised. The JPO started examinations based on the revised Guidelines by the end of April 2016.
According to Article 67 (2), when there is a period during which a patented invention cannot be implemented, the term of the patent right may be extended by filing a request for a patent term extension for a period, however, not to exceed five years. A patented invention may be unable to be implemented if it is necessary to obtain approvals or any other dispositions* as designated by the Cabinet Order under Article 67 (2) of the Patent Act.
According to Article 67ter(1)(i) of the Patent Act, the examiner shall reject a request for a pa-tent term extension, if the disposition designated by Cabinet Order under Article 67 (2) is not deemed to have been necessary for the patented invention to be implemented.
In the case, where there has been a prior disposition and there is a request for extension based on a further disposition (the current disposition), under the past guidelines a reason for refusal would have been issued according to Article 67ter(1)(i) of the Patent Act, if the scope of the current disposition could have been implemented within the scope of the prior disposition. The scope of a disposition is defined by the features of the pharmaceutical product or agricultural chemical, which correspond to the features of the granted claims, as well as is defined by the use of the pharmaceutical product or agricultural chemical (even if the use in question is not part of the features of the granted scope of protection).
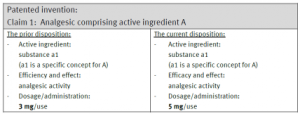
An example of a request for extensions of a pharmaceutical product that will be refused under the past guidelines is:
Although the current disposition has different dosage/administration, since the active ingredient and the use (i.e., the features of the granted claims) of the current disposition is the same as that of the prior disposition, the request for extension based on the current disposition would be rejected.
Supreme Court Decision
In the Supreme Court’s November 15, 2015 decision, the Court looked at the comparison of prior and current dispositions and held that the comparison shall no longer be based strictly on the features of the granted claims, but will be based on the “examination matters related directly to substantial identity” of the pharmaceutical product or agricultural chemical in accordance with the types of the patented invention.
Guidelines
The revised Guidelines follow the Supreme Court’s decision, and explain that when the manufacture and sale of pharmaceutical products or the manufacture or import of agricultural chemicals of the prior disposition include that of the current disposition, then there will be a reason to reject the application for extension based on the current disposition. Whether or not the manufacture and sale of pharmaceutical products or the manufacture or import of agricultural chemicals of a prior disposition include that of the current disposition is determined by comparing the two dispositions with respect to the “examination matters related directly to substantial identity” of the pharmaceutical product or agricultural chemical.
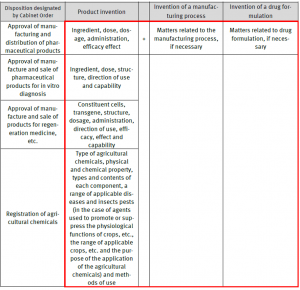
Example of “the Examination Matters Related Directly to Substantial Identity” from the revised Guidelines
The following examples is a copy from the revised Guideline and includes the particular disposition as well as “the examination matters related directly to the substantial identity” in accordance with types of the patented invention (see the matters in red marked frame):
Therefore, in the aforementioned example where the dosage/administration is different in the prior and current dispositions (i.e., increase to 5 mg/use), under the revised Guidelines, this is considered different examination matters and will not likely lead to a reason to reject this request for a patent term extension. The revised Guidelines also allow such differences in the dispositions be clarified in the request document, for example, by stating the differences in dosage and administration.
* The following are dispositions to be designated by Cabinet Order:
(i) a registration related to agricultural chemicals based on the provisions of the Agricultural Chemicals Regulation Act; and
(ii) an approval and certification based on the provisions of the “Pharmaceutical products and Medical devices Act” concerning pharmaceutical products, pharmaceutical products for in vitro diagnosis, products for regeneration medicine and the like.